abstract:
Would changing the monomer in the yeast compound affect the levels of CO2 produced? After running the complete lab, we have deducted that yeast with lipid produces the most CO2.
Would changing the monomer in the yeast compound affect the levels of CO2 produced? After running the complete lab, we have deducted that yeast with lipid produces the most CO2.
introduction:
We are doing this lab so that we may better understand whether or
not the monomer that is mixed into the yeast solution affects the amount of CO2
produced. To do this, we took four test tubes, each with a different monomer,
and set them up with a syringe stopper system, so that we could measure the amount
of CO2 that is produced.
hypothesis:
We
were asked to research yeast before we did this lab, and I found that yeast is
known to break down sugars and convert them to CO2. With this
knowledge, we can safely assume that the test tube with the sugar will produce
the most CO2.
procedure:- Set up four test tubes that all contain
- 1 gram of yeast
- 35 mL of water
- 0.1 g salt
- In each of the four test tubes, put one of each:
- Sugar
- Starch
- Protein
- Lipid
- Seal each of these test tubes with a stopped and syringe
- Read the amount of CO2 as shown on the syringe every minute for 5-10 minutes.
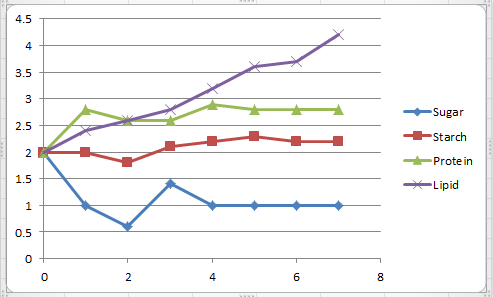
data:
Amount
of
|
CO2
Produced
|
(mL)
|
||
Sugar
|
Starch
|
Protein
|
Lipid
|
|
0
|
2
|
2
|
2
|
2
|
1
|
1
|
2
|
2.8
|
2.4
|
2
|
.6
|
1.8
|
2.6
|
2.6
|
3
|
1.4
|
2.1
|
2.6
|
2.8
|
4
|
1
|
2.2
|
2.9
|
3.2
|
5
|
1
|
2.3
|
2.8
|
3.6
|
6
|
1
|
2.2
|
2.8
|
3.7
|
7
|
1
|
2.2
|
2.8
|
4.2
|
I can safely say that our lab was a
failure. Our syringe stopper system failed to collect the CO2, and
we found error after error. We predicted that the test tube with the sugar would've had the greatest production of CO2, but we found that the
test tube with the lipid actually produced the most. It is possible that this
is true and that this is the correct outcome, there are so many sources of
error, and that I have simply labeled this lab as faulty data. First off, the
collection method for the CO2 was a bit sketchy. I am pretty sure
that only one of the systems worked correctly. This was a very crucial part of
the lab, and the fact that our equipment was faulty, completely undermined our
lab. Another source of error was the fact that I am human. My partner and I
were too slow at collecting the data right on the clock every minute, so we were
always off by a few seconds. The last source of error was the amount of the
specified monomer that was put into the test tube. We were asked to put in 1
gram, but my partner and I completely overlooked that specific amount and
decided on our own that we should use 0.5 grams of each. The lack of substance
could have tainted our data, because there wasn't enough of the substance to
keep the reaction going for the full seven minutes.